
Less is more?
Every so often is it worthwhile to look back at FDA to see what they had to say in a given year, and in addition, how they said it. One might not think that a large agency would vary much in terms of volume or style from year to year, but that is not the case.
How Many? This year the agency issued 190 releases, which is a substantial cutback from recent years in terms of sheer numbers. The last time the agency issued less than 200 releases to the public was back in 2017. There was a marked pickup during that when FDA was overseen by Dr. Scott Gottlieb who introduced frequent releases that were from his office directly, a practice that had prior to that time been very much an exception and not a rule. The peak year, 2020, represents another big increase and coincides with the advent of COVID when there was a great deal to say about the current environment.
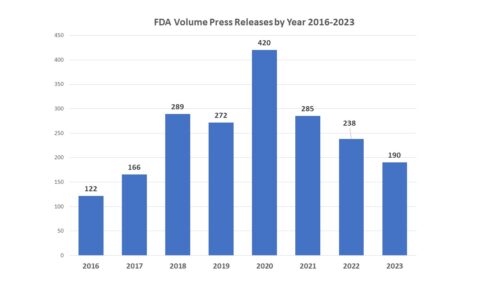
As noted in a posting on the half year check up, two factors figure in to the large drop. First, the COVID subject matter has slowed to a trickle. And second, the agency switched last year from issuing separate “FDA in Brief” notices to issuing releases called “FDA Roundup” that covers multiple items that, on their own, might not be press release-worthy in terms of news impact. In fact, the use of FDA Roundups appears to be on the rise, and this might be a factor in fewer traditional press releases coming out of FDA. In any case, no doubt about it, the agency volume of traditional releases has subsided greatly. If you factor out the FDA Roundups from the number for 2023, then the agency only sent out 102 traditional press releases.
What Did They Talk About? While in general, there was less talk, more of it was about approvals, which comes as little surprise perhaps given the fact that the number of new molecular entities (NME) approved by FDA during 2023 numbered 55, compared to only 37 the year before – a big jump and giving FDA much more to crow about. That said, only 25 releases were about the approval of new drugs, indicating that even though an approval may involve an NME, they don’t always issue a press release about it. The number of releases in relation to new gene therapies increased from 3 to 5, however it is worth noting that one of the 2023 releases actually addressed the approval of 2 new gene therapies in one release. Finally, no surprise here, the number of announcements about new vaccine approvals fell as the COVID pandemic entered a new phase.
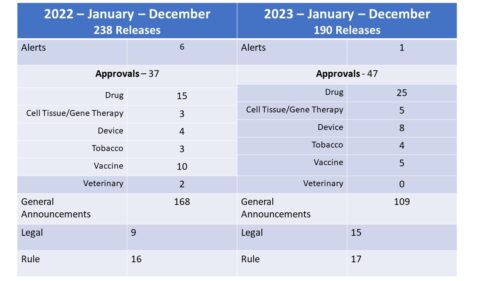
General Announcements include any releases not categorized in the other categories and also includes FDA Roundups. The communications about legal actions (Consent Decrees, Warnings, Seizures, e.g.) was up while announcements about new rules (Guidances, Regulations and Policy announcements) were nearly flat.
Did They Speak Spanish? The agency is spotty about issuing releases that are both in Spanish and English, but it would appear that this year there were more releases than ever issued in bilingual fashion. New approvals were the category that most often (but not always) in both languages. Overall, when you take out FDA Roundups which are always solely in English, it looks like 71 percent of the time, the agency went bilingual. Last year by my count it was only 55 percent. The number of releases was lower than last year, but the proportion of bilingual has gone up.
So far 2024 has started off very quietly. In January FDA issued only 2 press releases while the balance of communications were handled through a large number of “FDA Roundups”. A single NME was approved in January. Stay tuned to see how it looks in a mid-year assessment.


















